Our mission is to ensure the generation of accurate and precise findings.
Please enter subscribe form shortcode
Please enter instagram feed shortcode
Powder for solution for injection or infusion (Powder for injection/infusion).
Ceftriaxone injection is indicated in the treatment of UTI, lower respiratory tract infections, bacteraemia, septicaemia, meningitis, abdominal infections and infections caused by pseudomonas species.
Ceftriaxone for injection may be administered intravenously or intramuscularly.
Dosage and mode of administration should be determined by the severity of the infection, susceptibility of the causative organism and the patient’s condition. Under most circumstances a once-daily dose – or, in the specified indications, a single dose – will give satisfactory therapeutic results.
Pediatric patients
The recommended total daily dose is 50 to 75 mg/kg, given once daily or in equally divided doses every 12 hours or as directed by the Physician. The total daily dose should not exceed 2 grams.
In the treatment of meningitis, it is recommended that the initial therapeutic dose be 100 mg/kg (not to exceed 4 grams). Thereafter, a total daily dose of 100 mg/kg/day (not to exceed 4 grams daily) is recommended. The daily dose may be administered once a day (or in equally divided doses every 12 hours). The usual duration of therapy is 7 to 14 days.
Adults
The usual adult daily dose is 1 to 2 grams given once a day (or in equally divided doses twice a day) depending on the type and severity of infection. The total daily dose should not exceed 4 grams.
If Chlamydia trachomatis is a suspected pathogen, appropriate antichlamydial coverage should be added, because ceftriaxone sodium has no activity against this organism. For the treatment of uncomplicated gonococcal infections, a single intramuscular dose of 250 mg is recommended.
For preoperative use (surgical prophylaxis), a single dose of 1 gram administered intravenously 1/2 to 2 hours before surgery is recommended. Generally, Ceftriaxone therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required.
When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days.
Method of administration: Ceftriaxone injection may be administered by the I.V. or I.M. Route.
Directions for Use: The use of freshly prepared solutions is recommended. Ceftriaxone may be administered by deep I.M. injection, or as a slow I.V. injection/infusion, after reconstitution of the solution according to the directions given below:
I.V. injection should be administered over at least 2-4 minutes.
I.V. infusion should be over a period of 30 minutes.
Intravenous Administration: Ceftriaxone can be administered by intravenous infusion over at least 30 minutes (preferred route) or by slow intravenous injection over 5 minutes.
Intravenous intermittent injection should be given over 5 minutes preferably in larger veins. Intravenous doses of 50 mg/kg or more in infants and children up to 12 years of age should be given by infusion. In neonates, intravenous doses should be given over 60 minutes to reduce the potential risk of bilirubin encephalopathy (see section 4.3 and 4.4).
Intramuscular administration should be considered when the intravenous route is not possible or less appropriate for the patient. For doses greater than 2 g intravenous administration should be used.
For pre-operative prophylaxis of surgical site infections, ceftriaxone should be administered 30-90 minutes prior to surgery.
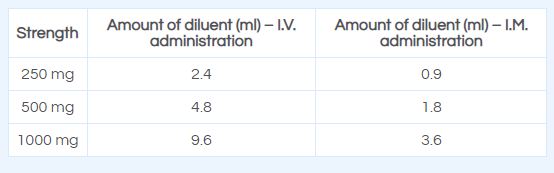
Intramuscular Administration: Reconstitute Ceftriaxone powder with the appropriate diluent. Inject diluent into vial, shake vial thoroughly to form solution. Withdraw entire contents of vial into syringe to equal total labeled dose.
After reconstitution, the solution should be administered by deep I.M. injection. Doses greater than 1 g should be divided and injected at more than one site. As with all I.M. preparations, ceftriaxone should be injected well within the body of a relatively large muscle; aspiration helps to avoid unintentional injection into a blood vessel.
Reconstitute ceftriaxone with the appropriate diluent, e.g. Water for Injection, IP, Normal Saline Water, or Dextrose Solutions.
Ceftriaxone injection is contraindicated in patients with known hypersensitivity to the active substance, to any other cephalosporin or to any of the excipients. History of severe hypersensitivity (e.g. anaphylactic reaction) to any other type of beta-lactam antibacterial agent (penicillins, monobactams and carbapenems).
Ceftriaxone is contraindicated in:
Premature neonates up to a postmenstrual age of 41 weeks (gestational age + chronological age)*
Full-term neonates (up to 28 days of age):
– With hyperbilirubinaemia, jaundice, or who are hypoalbuminaemic or acidotic because these are conditions in which bilirubin binding is likely to be impaired*
– If they require (or are expected to require) intravenous calcium treatment, or calcium-containing infusions due to the risk of precipitation of a ceftriaxone-calcium salt.
* In vitro studies have shown that ceftriaxone can displace bilirubin from its serum albumin binding sites leading to a possible risk of bilirubin encephalopathy in these patients.
Contraindications to lidocaine must be excluded before intramuscular injection of ceftriaxone when lidocaine solution is used as a solvent. Ceftriaxone solutions containing
lidocaine should never be administered intravenously.
Hypersensitivity reactions
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated. Before beginning treatment, it should be established whether the patient has a history of severe hypersensitivity reactions to ceftriaxone, to other cephalosporins or to any other type of beta-lactam agent. Caution should be used if ceftriaxone is given to patients with a history of non-severe hypersensitivity to other beta-lactam agents.
Severe cutaneous adverse reactions (Stevens Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) and drug reaction with eosinophilia and systemic symptoms (DRESS)) which can be life-threatening or fatal, have been reported in association with ceftriaxone treatment; however, the frequency of these events is not known.
Immune mediated haemolytic anaemia
An immune mediated haemolytic anaemia has been observed in patients receiving cephalosporin class antibacterials including Ceftriaxone. Severe cases of haemolytic anaemia, including fatalities, have been reported during Ceftriaxone treatment in both adults and children. If a patient develops anaemia while on ceftriaxone, the diagnosis of a cephalosporin-associated anaemia should be considered and ceftriaxone discontinued until the aetiology is determined.
Jarisch-Herxheimer reaction (JHR)
Some patients with spirochete infections may experience a Jarisch-Herxheimer reaction (JHR) shortly after ceftriaxone treatment is started. JHR is usually a self – limiting condition or can be managed by symptomatic treatment. The antibiotic treatment should not be discontinued if such reaction occurs.
Interaction with calcium containing products
Cases of fatal reactions with calcium-ceftriaxone precipitates in lungs and kidneys in premature and full-term neonates aged less than 1 month have been described. At least one of them had received ceftriaxone and calcium at different times and through different intravenous lines. In the available scientific data, there are no reports of confirmed intravascular precipitations in patients, other than neonates, treated with ceftriaxone and calcium-containing solutions or any other calcium-containing products. In vitro studies demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium compared to other age groups.
In patients of any age ceftriaxone must not be mixed or administered simultaneously with any calcium-containing intravenous solutions, even via different infusion lines or at different infusion sites. However, in patients older than 28 days of age ceftriaxone and calcium-containing solutions may be administered sequentially one after another if infusion lines at different sites are used or if the infusion lines are replaced or thoroughly flushed between infusions with physiological salt-solution to avoid precipitation. In patients requiring continuous infusion with calcium-containing total parenteral nutrition (TPN) solutions, healthcare professionals may wish to consider the use of alternative antibacterial treatments which do not carry a similar risk of precipitation. If the use of ceftriaxone is considered necessary in patients requiring continuous nutrition, TPN solutions and ceftriaxone can be administered simultaneously, albeit via different infusion lines at different sites. Alternatively, infusion of TPN solution could be stopped for the period of ceftriaxone infusion and the infusion lines flushed between solutions.
Paediatric population
Safety and effectiveness of Ceftriaxone in neonates, infants and children have been established for the dosages described under Posology and Method of Administration. Studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone is contraindicated in premature and full-term neonates at risk of developing bilirubin encephalopathy.
Long term treatment
During prolonged treatment complete blood count should be performed at regular intervals.
Colitis/Overgrowth of non-susceptible microorganisms
Antibacterial agent-associated colitis and pseudo-membranous colitis have been reported with nearly all antibacterial agents, including ceftriaxone, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea during or subsequent to the administration of ceftriaxone.
Discontinuation of therapy with ceftriaxone and the administration of specific treatment for Clostridium difficile should be considered. Medicinal products that inhibit peristalsis should not be given. Superinfections with non-susceptible micro-organisms may occur as with other antibacterial agents.
Severe renal and hepatic insufficiency
In severe renal and hepatic insufficiency, close clinical monitoring for safety and efficacy is advised.
Interference with serological testing
Interference with Coombs tests may occur, as Ceftriaxone may lead to false-positive test results. Ceftriaxone can also lead to false-positive test results for galactosaemia. Nonenzymatic methods for the glucose determination in urine may give false-positive results. Urine glucose determination during therapy with Ceftriaxone should be done enzymatically. The presence of ceftriaxone may falsely lower estimated blood glucose values obtained with some blood glucose monitoring systems. Please refer to instructions for use for each system. Alternative testing methods should be used if necessary.
Sodium
Each gram of ceftriaxone sodium contains approximately 3.6 mmol sodium. This should be taken into consideration in patients on a controlled sodium diet.
Antibacterial spectrum
Ceftriaxone has a limited spectrum of antibacterial activity and may not be suitable for use as a single agent for the treatment of some types of infections unless the pathogen has already been confirmed. In polymicrobial infections, where suspected pathogens include organisms resistant to ceftriaxone, administration of an additional antibiotic should be considered.
Use of lidocaine
In case a lidocaine solution is used as a solvent, ceftriaxone solutions must only be used for intramuscular injection. Contraindications to lidocaine, warnings and other relevant information as detailed in the Summary of Product Characteristics of lidocaine must be considered before use. The lidocaine solution should never be administered intravenously.
Biliary lithiasis
When shadows are observed on sonograms, consideration should be given to the possibility of precipitates of calcium ceftriaxone. Shadows, which have been mistaken for gallstones, have been detected on sonograms of the gallbladder and have been observed more frequently at ceftriaxone doses of 1 g per day and above. Caution should be particularly considered in the paediatric population. Such precipitates disappear after discontinuation of ceftriaxone therapy. Rarely precipitates of calcium ceftriaxone have been associated with symptoms. In symptomatic cases, conservative nonsurgical management is recommended and discontinuation of ceftriaxone treatment should be considered by the physician based on specific benefit risk assessment.
Biliary stasis
Cases of pancreatitis, possibly of biliary obstruction aetiology, have been reported in patients treated with Ceftriaxone. Most patients presented with risk factors for biliary stasis and biliary sludge e.g. preceding major therapy, severe illness and total parenteral nutrition. A trigger or cofactor of Ceftriaxone-related biliary precipitation cannot be ruled out.
Renal lithiasis
Cases of renal lithiasis have been reported, which is reversible upon discontinuation of ceftriaxone. In symptomatic cases, sonography should be performed. Use in patients with history of renal lithiasis or with hypercalciuria should be considered by the physician based on specific benefit risk assessment.
Pregnancy
Teratogenic Effects: Pregnancy Category B. Ceftriaxone crosses the placental barrier. There are limited amounts of data from the use of ceftriaxone in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to embryonal/foetal, perinatal and postnatal development. Ceftriaxone should only be administered during pregnancy and in particular in the first trimester of pregnancy if the benefit outweighs the risk.
Breastfeeding
Ceftriaxone is excreted into human milk in low concentrations but at therapeutic doses of ceftriaxone no effects on the breastfed infants are anticipated. However, a risk of diarrhoea and fungal infection of the mucous membranes cannot be excluded. The possibility of sensitisation should be taken into account. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from ceftriaxone therapy, taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman.
Pediatric Use
Safety and effectiveness of Ceftriaxone in neonates, infants and pediatric patients have been established for the dosages described in the Dosage and Administration section. In vitro studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone should not be administered to hyperbilirubinemic neonates, especially prematures (see Contraindications).
Geriatric Use
Of the total number of subjects in clinical studies of Ceftriaxone, 32% were 60 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetics of ceftriaxone were only minimally altered in geriatric patients compared to healthy adult subjects and dosage adjustments are not necessary for geriatric patients with ceftriaxone dosages up to 2 grams per day.
There are no data to indicate any effect on a person’s ability to drive or use machines.
Fixed drug eruption (FDE) has been reported with cephalosporin class formulations.
Ceftriaxone induced Stevens-Johnson Syndrome (SJS).
Ceftriaxone is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to Ceftriaxone therapy or of uncertain etiology, were observed:
Local Reactions: Pain, induration and tenderness was 1% overall. Phlebitis was reported in <1% after IV administration. The incidence of warmth, tightness or induration was 17% (3/17) after IM administration of 350 mg/mL and 5% (1/20) after IM administration of 250 mg/mL.
Hypersensitivity: Rash (1.7%). Less frequently reported (<1%) were pruritus, fever or chills.
Hematologic: Eosinophilia (6%), thrombocytosis (5.1%) and leukopenia (2.1%). Less frequently reported (<1%) were anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia and prolongation of the prothrombin time.
Gastrointestinal: Diarrhea (2.7%). Less frequently reported (<1%) were nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment.
Hepatic: Elevations of SGOT (3.1%) or SGPT (3.3%). Less frequently reported (<1%) were elevations of alkaline phosphatase and bilirubin.
Renal: Elevations of the BUN (1.2%). Less frequently reported (<1%) were elevations of creatinine and the presence of casts in the urine.
Central Nervous System: Headache or dizziness were reported occasionally (<1%).
Genitourinary: Moniliasis or vaginitis were reported occasionally (<1%).
Miscellaneous: Diaphoresis and flushing were reported occasionally (<1%).
Other rarely observed adverse reactions (<0.1%) include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
Post-marketing Experience
In addition to the adverse reactions reported during clinical trials, the following adverse experiences have been reported during clinical practice in patients treated with Ceftriaxone. Data are generally insufficient to allow an estimate of incidence or to establish causation.
A small number of cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving Ceftriaxone and calcium-containing fluids. In some of these cases, the same intravenous infusion line was used for both Ceftriaxone and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. At least one fatality has been reported in a neonate in whom Ceftriaxone and calcium-containing fluids were administered at different time points via different intravenous lines; no crystalline material was observed at autopsy in this neonate. There have been no similar reports in patients other than neonates.
Gastrointestinal: Stomatitis and glossitis.
Genitourinary: Oliguria.
Dermatologic: Exanthema, allergic dermatitis, urticaria, oedema. As with many medications, isolated cases of severe cutaneous adverse reactions (erythema multiforme, Stevens-Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) have been reported.
Cephalosporin-class adverse reactions
In addition to the adverse reactions listed above, which have been observed in patients treated with ceftriaxone, the following adverse reactions and altered laboratory test results have been reported for cephalosporin-class antibiotics:
Adverse Reactions: Allergic reactions, drug fever, serum sickness-like reaction, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction, including cholestasis, aplastic anaemia, haemorrhage, and super-infection.
Altered Laboratory Tests: Positive direct Coombs’ test, false-positive test for urinary glucose, and elevated LDH.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
In overdose, the symptoms of nausea, vomiting and diarrhoea can occur. Ceftriaxone concentrations cannot be reduced by haemodialysis or peritoneal dialysis. There is no specific antidote. Treatment is symptomatic.
Pharmacodynamic properties
Pharmacotherapeutic group: Antibacterials for systemic use, Third-generation cephalosporins.
Ceftriaxone is a 2-aminothiazolyl methoxyimino third-generation cephalosporin derivative. Ceftriaxone offers good activity against gram-negative organisms with reasonable activity against gram-positive organisms.
Ceftriaxone, a bactericidal antimicrobial, inhibits bacterial wall synthesis of actively dividing cells by binding to one or more penicillin bind proteins (PBPs). These proteins are associated with the bacterial cell membrane and probably serve as synthesis. The result is formation of a defective cell wall that is osmotically unstable. Bacterial species have a unique set of PBPs. The affinity pattern of ceftriaxone for the PBPs for different bacterial species affects the drug’s antimicrobial spectrum of activity. It is also felt that cephalosporins, as well as penicillins, may increase the breakdown of the cell wall of bacteria by decreasing the availability of an inhibitor of murein hydrolase, an enzyme involved in cell division. If unimposed, this enzyme can destroy the integrity of the cell wall. In the presence of beta-lactamase bacteria, including penicillinases and cephalosporinases, ceftriaxone maintains a high degree of stability.
Pharmacokinetics properties
Absorption
Following intramuscular injection, mean peak plasma ceftriaxone levels are approximately half those observed after intravenous administration of an equivalent dose. The maximum plasma concentration after a single intramuscular dose of 1 g is about 81 mg/l and is reached in 2 – 3 hours after administration. The area under the plasma concentration-time curve after intramuscular administration is equivalent to that after intravenous administration of an equivalent dose.
After intravenous bolus administration of ceftriaxone 500 mg and 1 g, mean peak plasma ceftriaxone levels are approximately 120 and 200 mg/l respectively. After intravenous infusion of ceftriaxone 500 mg, 1 g and 2 g, the plasma ceftriaxone levels are approximately 80, 150 and 250 mg/l respectively.
Adults:
Average plasma concentrations of ceftriaxone after administration of a single dose are given in the table below:
Table 1: Ceftriaxone Plasma Concentrations after Single-Dose Administration
Powder for solution for injection or infusion (Powder for injection/infusion).
Ceftriaxone injection is indicated in the treatment of UTI, lower respiratory tract infections, bacteraemia, septicaemia, meningitis, abdominal infections and infections caused by pseudomonas species.
Ceftriaxone for injection may be administered intravenously or intramuscularly.
Dosage and mode of administration should be determined by the severity of the infection, susceptibility of the causative organism and the patient’s condition. Under most circumstances a once-daily dose – or, in the specified indications, a single dose – will give satisfactory therapeutic results.
Pediatric patients
The recommended total daily dose is 50 to 75 mg/kg, given once daily or in equally divided doses every 12 hours or as directed by the Physician. The total daily dose should not exceed 2 grams.
In the treatment of meningitis, it is recommended that the initial therapeutic dose be 100 mg/kg (not to exceed 4 grams). Thereafter, a total daily dose of 100 mg/kg/day (not to exceed 4 grams daily) is recommended. The daily dose may be administered once a day (or in equally divided doses every 12 hours). The usual duration of therapy is 7 to 14 days.
Adults
The usual adult daily dose is 1 to 2 grams given once a day (or in equally divided doses twice a day) depending on the type and severity of infection. The total daily dose should not exceed 4 grams.
If Chlamydia trachomatis is a suspected pathogen, appropriate antichlamydial coverage should be added, because ceftriaxone sodium has no activity against this organism. For the treatment of uncomplicated gonococcal infections, a single intramuscular dose of 250 mg is recommended.
For preoperative use (surgical prophylaxis), a single dose of 1 gram administered intravenously 1/2 to 2 hours before surgery is recommended. Generally, Ceftriaxone therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required.
When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days.
Method of administration: Ceftriaxone injection may be administered by the I.V. or I.M. Route.
Directions for Use: The use of freshly prepared solutions is recommended. Ceftriaxone may be administered by deep I.M. injection, or as a slow I.V. injection/infusion, after reconstitution of the solution according to the directions given below:
I.V. injection should be administered over at least 2-4 minutes.
I.V. infusion should be over a period of 30 minutes.
Intravenous Administration: Ceftriaxone can be administered by intravenous infusion over at least 30 minutes (preferred route) or by slow intravenous injection over 5 minutes.
Intravenous intermittent injection should be given over 5 minutes preferably in larger veins. Intravenous doses of 50 mg/kg or more in infants and children up to 12 years of age should be given by infusion. In neonates, intravenous doses should be given over 60 minutes to reduce the potential risk of bilirubin encephalopathy (see section 4.3 and 4.4).
Intramuscular administration should be considered when the intravenous route is not possible or less appropriate for the patient. For doses greater than 2 g intravenous administration should be used.
For pre-operative prophylaxis of surgical site infections, ceftriaxone should be administered 30-90 minutes prior to surgery.
Intramuscular Administration: Reconstitute Ceftriaxone powder with the appropriate diluent. Inject diluent into vial, shake vial thoroughly to form solution. Withdraw entire contents of vial into syringe to equal total labeled dose.
After reconstitution, the solution should be administered by deep I.M. injection. Doses greater than 1 g should be divided and injected at more than one site. As with all I.M. preparations, ceftriaxone should be injected well within the body of a relatively large muscle; aspiration helps to avoid unintentional injection into a blood vessel.
Reconstitute ceftriaxone with the appropriate diluent, e.g. Water for Injection, IP, Normal Saline Water, or Dextrose Solutions.

Ceftriaxone injection is contraindicated in patients with known hypersensitivity to the active substance, to any other cephalosporin or to any of the excipients. History of severe hypersensitivity (e.g. anaphylactic reaction) to any other type of beta-lactam antibacterial agent (penicillins, monobactams and carbapenems).
Ceftriaxone is contraindicated in:
Premature neonates up to a postmenstrual age of 41 weeks (gestational age + chronological age)*
Full-term neonates (up to 28 days of age):
– With hyperbilirubinaemia, jaundice, or who are hypoalbuminaemic or acidotic because these are conditions in which bilirubin binding is likely to be impaired*
– If they require (or are expected to require) intravenous calcium treatment, or calcium-containing infusions due to the risk of precipitation of a ceftriaxone-calcium salt.
* In vitro studies have shown that ceftriaxone can displace bilirubin from its serum albumin binding sites leading to a possible risk of bilirubin encephalopathy in these patients.
Contraindications to lidocaine must be excluded before intramuscular injection of ceftriaxone when lidocaine solution is used as a solvent. Ceftriaxone solutions containing
lidocaine should never be administered intravenously.
Hypersensitivity reactions
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated. Before beginning treatment, it should be established whether the patient has a history of severe hypersensitivity reactions to ceftriaxone, to other cephalosporins or to any other type of beta-lactam agent. Caution should be used if ceftriaxone is given to patients with a history of non-severe hypersensitivity to other beta-lactam agents.
Severe cutaneous adverse reactions (Stevens Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) and drug reaction with eosinophilia and systemic symptoms (DRESS)) which can be life-threatening or fatal, have been reported in association with ceftriaxone treatment; however, the frequency of these events is not known.
Immune mediated haemolytic anaemia
An immune mediated haemolytic anaemia has been observed in patients receiving cephalosporin class antibacterials including Ceftriaxone. Severe cases of haemolytic anaemia, including fatalities, have been reported during Ceftriaxone treatment in both adults and children. If a patient develops anaemia while on ceftriaxone, the diagnosis of a cephalosporin-associated anaemia should be considered and ceftriaxone discontinued until the aetiology is determined.
Jarisch-Herxheimer reaction (JHR)
Some patients with spirochete infections may experience a Jarisch-Herxheimer reaction (JHR) shortly after ceftriaxone treatment is started. JHR is usually a self – limiting condition or can be managed by symptomatic treatment. The antibiotic treatment should not be discontinued if such reaction occurs.
Interaction with calcium containing products
Cases of fatal reactions with calcium-ceftriaxone precipitates in lungs and kidneys in premature and full-term neonates aged less than 1 month have been described. At least one of them had received ceftriaxone and calcium at different times and through different intravenous lines. In the available scientific data, there are no reports of confirmed intravascular precipitations in patients, other than neonates, treated with ceftriaxone and calcium-containing solutions or any other calcium-containing products. In vitro studies demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium compared to other age groups.
In patients of any age ceftriaxone must not be mixed or administered simultaneously with any calcium-containing intravenous solutions, even via different infusion lines or at different infusion sites. However, in patients older than 28 days of age ceftriaxone and calcium-containing solutions may be administered sequentially one after another if infusion lines at different sites are used or if the infusion lines are replaced or thoroughly flushed between infusions with physiological salt-solution to avoid precipitation. In patients requiring continuous infusion with calcium-containing total parenteral nutrition (TPN) solutions, healthcare professionals may wish to consider the use of alternative antibacterial treatments which do not carry a similar risk of precipitation. If the use of ceftriaxone is considered necessary in patients requiring continuous nutrition, TPN solutions and ceftriaxone can be administered simultaneously, albeit via different infusion lines at different sites. Alternatively, infusion of TPN solution could be stopped for the period of ceftriaxone infusion and the infusion lines flushed between solutions.
Paediatric population
Safety and effectiveness of Ceftriaxone in neonates, infants and children have been established for the dosages described under Posology and Method of Administration. Studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone is contraindicated in premature and full-term neonates at risk of developing bilirubin encephalopathy.
Long term treatment
During prolonged treatment complete blood count should be performed at regular intervals.
Colitis/Overgrowth of non-susceptible microorganisms
Antibacterial agent-associated colitis and pseudo-membranous colitis have been reported with nearly all antibacterial agents, including ceftriaxone, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea during or subsequent to the administration of ceftriaxone.
Discontinuation of therapy with ceftriaxone and the administration of specific treatment for Clostridium difficile should be considered. Medicinal products that inhibit peristalsis should not be given. Superinfections with non-susceptible micro-organisms may occur as with other antibacterial agents.
Severe renal and hepatic insufficiency
In severe renal and hepatic insufficiency, close clinical monitoring for safety and efficacy is advised.
Interference with serological testing
Interference with Coombs tests may occur, as Ceftriaxone may lead to false-positive test results. Ceftriaxone can also lead to false-positive test results for galactosaemia. Nonenzymatic methods for the glucose determination in urine may give false-positive results. Urine glucose determination during therapy with Ceftriaxone should be done enzymatically. The presence of ceftriaxone may falsely lower estimated blood glucose values obtained with some blood glucose monitoring systems. Please refer to instructions for use for each system. Alternative testing methods should be used if necessary.
Sodium
Each gram of ceftriaxone sodium contains approximately 3.6 mmol sodium. This should be taken into consideration in patients on a controlled sodium diet.
Antibacterial spectrum
Ceftriaxone has a limited spectrum of antibacterial activity and may not be suitable for use as a single agent for the treatment of some types of infections unless the pathogen has already been confirmed. In polymicrobial infections, where suspected pathogens include organisms resistant to ceftriaxone, administration of an additional antibiotic should be considered.
Use of lidocaine
In case a lidocaine solution is used as a solvent, ceftriaxone solutions must only be used for intramuscular injection. Contraindications to lidocaine, warnings and other relevant information as detailed in the Summary of Product Characteristics of lidocaine must be considered before use. The lidocaine solution should never be administered intravenously.
Biliary lithiasis
When shadows are observed on sonograms, consideration should be given to the possibility of precipitates of calcium ceftriaxone. Shadows, which have been mistaken for gallstones, have been detected on sonograms of the gallbladder and have been observed more frequently at ceftriaxone doses of 1 g per day and above. Caution should be particularly considered in the paediatric population. Such precipitates disappear after discontinuation of ceftriaxone therapy. Rarely precipitates of calcium ceftriaxone have been associated with symptoms. In symptomatic cases, conservative nonsurgical management is recommended and discontinuation of ceftriaxone treatment should be considered by the physician based on specific benefit risk assessment.
Biliary stasis
Cases of pancreatitis, possibly of biliary obstruction aetiology, have been reported in patients treated with Ceftriaxone. Most patients presented with risk factors for biliary stasis and biliary sludge e.g. preceding major therapy, severe illness and total parenteral nutrition. A trigger or cofactor of Ceftriaxone-related biliary precipitation cannot be ruled out.
Renal lithiasis
Cases of renal lithiasis have been reported, which is reversible upon discontinuation of ceftriaxone. In symptomatic cases, sonography should be performed. Use in patients with history of renal lithiasis or with hypercalciuria should be considered by the physician based on specific benefit risk assessment.
Pregnancy
Teratogenic Effects: Pregnancy Category B. Ceftriaxone crosses the placental barrier. There are limited amounts of data from the use of ceftriaxone in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to embryonal/foetal, perinatal and postnatal development. Ceftriaxone should only be administered during pregnancy and in particular in the first trimester of pregnancy if the benefit outweighs the risk.
Breastfeeding
Ceftriaxone is excreted into human milk in low concentrations but at therapeutic doses of ceftriaxone no effects on the breastfed infants are anticipated. However, a risk of diarrhoea and fungal infection of the mucous membranes cannot be excluded. The possibility of sensitisation should be taken into account. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from ceftriaxone therapy, taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman.
Pediatric Use
Safety and effectiveness of Ceftriaxone in neonates, infants and pediatric patients have been established for the dosages described in the Dosage and Administration section. In vitro studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone should not be administered to hyperbilirubinemic neonates, especially prematures (see Contraindications).
Geriatric Use
Of the total number of subjects in clinical studies of Ceftriaxone, 32% were 60 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetics of ceftriaxone were only minimally altered in geriatric patients compared to healthy adult subjects and dosage adjustments are not necessary for geriatric patients with ceftriaxone dosages up to 2 grams per day.
There are no data to indicate any effect on a person’s ability to drive or use machines.
Fixed drug eruption (FDE) has been reported with cephalosporin class formulations.
Ceftriaxone induced Stevens-Johnson Syndrome (SJS).
Ceftriaxone is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to Ceftriaxone therapy or of uncertain etiology, were observed:
Local Reactions: Pain, induration and tenderness was 1% overall. Phlebitis was reported in <1% after IV administration. The incidence of warmth, tightness or induration was 17% (3/17) after IM administration of 350 mg/mL and 5% (1/20) after IM administration of 250 mg/mL.
Hypersensitivity: Rash (1.7%). Less frequently reported (<1%) were pruritus, fever or chills.
Hematologic: Eosinophilia (6%), thrombocytosis (5.1%) and leukopenia (2.1%). Less frequently reported (<1%) were anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia and prolongation of the prothrombin time.
Gastrointestinal: Diarrhea (2.7%). Less frequently reported (<1%) were nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment.
Hepatic: Elevations of SGOT (3.1%) or SGPT (3.3%). Less frequently reported (<1%) were elevations of alkaline phosphatase and bilirubin.
Renal: Elevations of the BUN (1.2%). Less frequently reported (<1%) were elevations of creatinine and the presence of casts in the urine.
Central Nervous System: Headache or dizziness were reported occasionally (<1%).
Genitourinary: Moniliasis or vaginitis were reported occasionally (<1%).
Miscellaneous: Diaphoresis and flushing were reported occasionally (<1%).
Other rarely observed adverse reactions (<0.1%) include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
Post-marketing Experience
In addition to the adverse reactions reported during clinical trials, the following adverse experiences have been reported during clinical practice in patients treated with Ceftriaxone. Data are generally insufficient to allow an estimate of incidence or to establish causation.
A small number of cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving Ceftriaxone and calcium-containing fluids. In some of these cases, the same intravenous infusion line was used for both Ceftriaxone and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. At least one fatality has been reported in a neonate in whom Ceftriaxone and calcium-containing fluids were administered at different time points via different intravenous lines; no crystalline material was observed at autopsy in this neonate. There have been no similar reports in patients other than neonates.
Gastrointestinal: Stomatitis and glossitis.
Genitourinary: Oliguria.
Dermatologic: Exanthema, allergic dermatitis, urticaria, oedema. As with many medications, isolated cases of severe cutaneous adverse reactions (erythema multiforme, Stevens-Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) have been reported.
Cephalosporin-class adverse reactions
In addition to the adverse reactions listed above, which have been observed in patients treated with ceftriaxone, the following adverse reactions and altered laboratory test results have been reported for cephalosporin-class antibiotics:
Adverse Reactions: Allergic reactions, drug fever, serum sickness-like reaction, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction, including cholestasis, aplastic anaemia, haemorrhage, and super-infection.
Altered Laboratory Tests: Positive direct Coombs’ test, false-positive test for urinary glucose, and elevated LDH.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
In overdose, the symptoms of nausea, vomiting and diarrhoea can occur. Ceftriaxone concentrations cannot be reduced by haemodialysis or peritoneal dialysis. There is no specific antidote. Treatment is symptomatic.
Pharmacodynamic properties
Pharmacotherapeutic group: Antibacterials for systemic use, Third-generation cephalosporins.
Ceftriaxone is a 2-aminothiazolyl methoxyimino third-generation cephalosporin derivative. Ceftriaxone offers good activity against gram-negative organisms with reasonable activity against gram-positive organisms.
Ceftriaxone, a bactericidal antimicrobial, inhibits bacterial wall synthesis of actively dividing cells by binding to one or more penicillin bind proteins (PBPs). These proteins are associated with the bacterial cell membrane and probably serve as synthesis. The result is formation of a defective cell wall that is osmotically unstable. Bacterial species have a unique set of PBPs. The affinity pattern of ceftriaxone for the PBPs for different bacterial species affects the drug’s antimicrobial spectrum of activity. It is also felt that cephalosporins, as well as penicillins, may increase the breakdown of the cell wall of bacteria by decreasing the availability of an inhibitor of murein hydrolase, an enzyme involved in cell division. If unimposed, this enzyme can destroy the integrity of the cell wall. In the presence of beta-lactamase bacteria, including penicillinases and cephalosporinases, ceftriaxone maintains a high degree of stability.
Pharmacokinetics properties
Absorption
Following intramuscular injection, mean peak plasma ceftriaxone levels are approximately half those observed after intravenous administration of an equivalent dose. The maximum plasma concentration after a single intramuscular dose of 1 g is about 81 mg/l and is reached in 2 – 3 hours after administration. The area under the plasma concentration-time curve after intramuscular administration is equivalent to that after intravenous administration of an equivalent dose.
After intravenous bolus administration of ceftriaxone 500 mg and 1 g, mean peak plasma ceftriaxone levels are approximately 120 and 200 mg/l respectively. After intravenous infusion of ceftriaxone 500 mg, 1 g and 2 g, the plasma ceftriaxone levels are approximately 80, 150 and 250 mg/l respectively.
Adults:
Average plasma concentrations of ceftriaxone after administration of a single dose are given in the table below:
Table 1: Ceftriaxone Plasma Concentrations after Single-Dose Administration
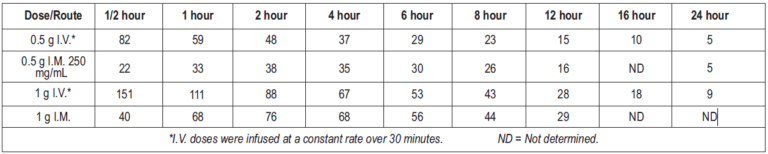
Ceftriaxone was completely absorbed following I.M. administration, with mean maximum plasma concentrations occurring between 2 and 3 hours post-dose. Multiple I.V. or I.M. doses ranging from 0.5 to 2 g at 12- to 24-hour intervals resulted in 15-36% accumulation of ceftriaxone above single-dose values.
Ceftriaxone concentrations in urine are shown in Table 2.
Table 2: Urinary Concentrations of Ceftriaxone after Single-Dose Administration)
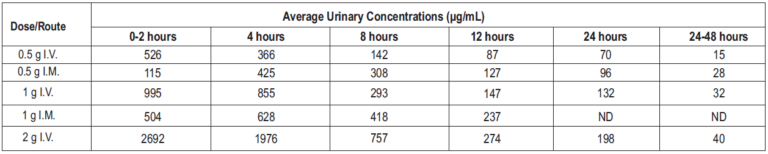
Altogether, 3367% of the ceftriaxone dose is excreted in the urine as unchanged drug and the remainder is excreted in the bile and the faeces. After a 1 g I.V. dose, average concentrations of ceftriaxone achieved in the gall bladder bile is 581 mcg/ml, 788 mcg/ml in the common bile duct, 898 mcg/ml in the cystic bile duct, 78.2 μg/g in the gall bladder wall and 62.1 μg/ml in concurrent plasma.
Distribution
The volume of distribution of ceftriaxone is 7-12L. Concentrations well above the minimal inhibitory concentrations of most relevant pathogens are detectable in tissue including lung, heart, biliary tract/liver, tonsil, middle ear and nasal mucosa, bone, and in cerebrospinal, pleural, prostatic and synovial fluids. An 8 – 15 % increase in mean peak plasma concentration (Cmax) is seen on repeated administration; steady state is reached in most cases within 48 – 72 hours depending on the route of administration. Ceftriaxone is reversibly bound to albumin. Plasma protein binding is about 95 % at plasma concentrations below 100 mg/l. Binding is saturable and the bound portion decreases with rising concentration (up to 85 % at a plasma concentration of 300 mg/l).
Biotransformation
Ceftriaxone is not metabolised systemically; but is converted to inactive metabolites by the gut flora.
Elimination
Plasma clearance of total ceftriaxone (bound and unbound) is 10 – 22 ml/min. Renal clearance is 5 – 12 ml/min. 50 – 60 % of ceftriaxone is excreted unchanged in the urine, primarily by glomerular filtration, while 40 – 50 % is excreted unchanged in the bile. The elimination half-life of total ceftriaxone in adults is about 8 hours.
Based on literature reports, ceftriaxone is not compatible with amsacrine, vancomycin, fluconazole and aminoglycosides and labetalol.
Solutions containing ceftriaxone should not be mixed with or added to other agents. In particular, diluents containing calcium, (e.g. Ringer’s solution, Hartmann’s solution) should not be used to reconstitute ceftriaxone vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Ceftriaxone must not be mixed or administered simultaneously with calcium containing solutions including total parenteral nutrition.
If treatment with a combination of another antibiotic with Ceftriaxone is intended, administration should not occur in the same syringe or in the same infusion solution.
This medicinal product must not be mixed with other medicinal products. From a microbiological point of view, once opened, the product should be used immediately. If not used immediately.
WhatsApp us
