Our mission is to ensure the generation of accurate and precise findings.
Please enter subscribe form shortcode
Please enter instagram feed shortcode
Film coated tablet
Acute bronchitis, exacerbations of chronic bronchitis, bronchiolitis pneumonia, sinusitis, recurrent chronic tonsillitis, pharyngitis, acute otitis.
Posology
The tablets should be taken with food to enhance absorption.
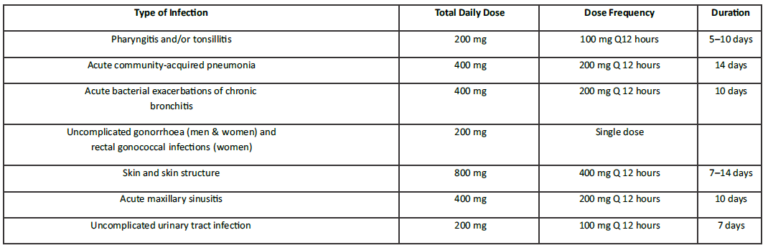
The recommended dosages, duration of treatment and applicable patient population are as described in the following table:
Adults and adolescents (aged 12 years and older):
It is contraindicated in patients with a known allergy to cefpodoxime or to the cephalosporin group of antibiotics or to any of its components.
It is also contraindicated in patients with previous history of immediate and / or severe hypersensitivity reaction (anaphylaxis) to penicillin or other beta-lactam antibiotic.
Warnings
Before therapy with cefpodoxime proxetil is instituted, a detailed inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cefpodoxime, other cephalosporins, penicillins, or other drugs. If cefpodoxime is to be administered to penicillin-sensitive patients, caution should be exercised because crosshypersensitivity among beta-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions have been reported. In case of severe hypersensitivity reactions, treatment with cefpodoxime must be discontinued immediately and adequate emergency measures must be initiated. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures, including oxygen, intravenous fluids, intravenous antihistamine, and airway management, as clinically indicated. Caution should be used if cefpodoxime is given to patients with a history of non-severe hypersensitivity to other beta-lactam agents.
Precautions
In patients with transient or persistent reduction in urinary output due to renal impairment, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. Discontinuation of therapy with cefpodoxime and the administration of specific treatment for Clostridium difficile should be considered. Medicinal products that inhibit peristalsis should not be given.
Information for Patients
Patients should be counselled that antibacterial drugs including cefpodoxime proxetil should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefpodoxime proxetil is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefpodoxime proxetil or other antibacterial drugs in the future.
Diarrhoea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Antacids
Concomitant administration of high doses of antacids (sodium bicarbonate and aluminium hydroxide) or H2 blockers reduces peak plasma levels by 24–42% and the extent of absorption by 27–32%, respectively. The rate of absorption is not altered by these concomitant medications. Oral anticholinergics (eg, propantheline) delay peak plasma levels (47% increase in Tmax), but do not affect the extent of absorption (AUC).
Probenecid
As with other beta-lactam antibiotics, renal excretion of cefpodoxime was inhibited by probenecid and resulted in an approximately 31% increase in AUC and a 20% increase in peak cefpodoxime plasma levels.
Nephrotoxic Drugs
Although nephrotoxicity has not been noted when cefpodoxime proxetil was given alone, close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential.
Food
The bioavailability increases if Cefpodoxime tablets are administered during meals.
Oral Anticoagulants
Simultaneous administration of cefpodoxime with warfarin may augment its anticoagulant effects. There have been many reports of increases in oral anticoagulant activity in patients receiving antibacterial agents, including cephalosporins. The risk may vary with the underlying infection, age and general status of the patient so that the contribution of the cephalosporins to the increase in INR (international normalised ratio) is difficult to assess. It is recommended that the INR should be monitored frequently during and shortly after coadministration of cefpodoxime with an oral anticoagulant agent.
Drug/Laboratory Test Interactions
Interaction with Laboratory tests- A false positive reaction for glucose in the urine may occur with Benedict’s or Fehling’s solutions or with copper sulphate test tablets, but not with tests based on enzymatic glucose oxidase reactions.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine. Cephalosporins, including cefpodoxime proxetil, are known to occasionally induce a positive direct Coombs’ test.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
There are no adequate and well-controlled studies of cefpodoxime proxetil use in pregnant women. Because animal reproduction studies are not always predictive of human response, Cefpodoxime tablets should be used during pregnancy only if clearly needed.
Breast-feeding
Because of the potential for serious reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Cefpodoxime is excreted in breast milk in small amounts. Cefpodoxime may be used during breastfeeding. Continuation of breastfeeding should be questioned in case of diarrhoea or mucosal fungus infection in the breastfed infant. The possibility of sensitisation should be borne in mind.
Paediatric population
Safety and efficacy in infants less than 2 months of age have not been established.
Elderly patients
Dose adjustment in elderly patients with normal renal function is not necessary.
Hepatic impairment
No dose adjustment is recommended for patients with hepatic insufficiency.
Renal impairment
For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to q24 hours. In patients maintained on hemodialysis, the dose frequency should be three times/week after hemodialysis. No data are available in case of pediatric patients with impaired renal function.
Dizziness has been reported during treatment with cefpodoxime and may affect the ability to drive and use machines.
Fixed drug eruption (FDE) has been reported with cephalosporin class formulations.
Cefpodoxime is well tolerated. Most common gastrointestinal adverse effects seen is diarrhoea, vomiting and abdominal pain.
Incidence greater than 1%
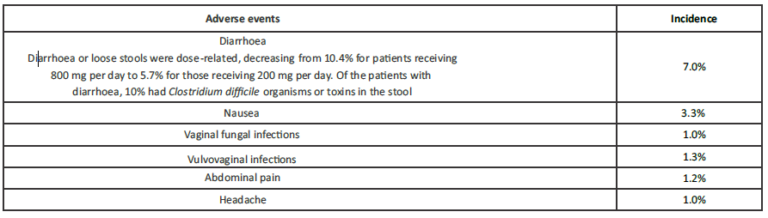
Incidence less than 1%
Adverse events, by body system in decreasing order, considered possibly or probably related to cefpodoxime proxetil and which occurred in less than 1% of patients, were as follows:
Body: Fungal infections, abdominal distention, malaise, fatigue, asthenia, fever, chest pain, back pain, chills, generalized pain, abnormal microbiological tests, moniliasis, abscess, allergic reaction, facial edema, bacterial infections, parasitic infections, localized edema, and localized pain.
Cardiovascular: congestive heart failure, migraine, palpitations, vasodilation, hematoma, hypertension, hypotension.
Digestive: vomiting, dyspepsia, dry mouth, flatulence, decreased appetite, constipation, oral moniliasis, anorexia, eructation, gastritis, mouth ulcers, gastrointestinal disorders, rectal disorders, tongue disorders, tooth disorders, increased thirst, oral lesions, tenesmus, dry throat, toothache.
Blood and Lymphatic: anaemia.
Metabolic and Nutritional: Dehydration, gout, peripheral edema, weight increase. Increased SGPT, ASAT, ALAT and alkaline phosphatase and/or bilirubin, liver damage
Musculoskeletal: Myalgia.
Nervous: dizziness, insomnia, somnolence, anxiety, shakiness, nervousness, cerebral infarction, change in dreams, impaired concentration, confusion, nightmares, paresthesia, vertigo.
Respiratory: asthma, cough, epistaxis, rhinitis, wheezing, bronchitis, dyspnea, pleural effusion, pneumonia, sinusitis.
Skin: Urticaria, rash, pruritus non-application site, diaphoresis, maculopapular rash, fungal dermatitis, desquamation, dry skin non-application site, hair loss, vesiculobullous rash, sunburn, hypersensitivity mucocutaneous reactions.
Special Senses: Taste alterations, eye irritation, taste loss, tinnitus.
Urogenital: Hematuria, urinary tract infections, metrorrhagia, dysuria, urinary frequency, nocturia, penile infection, proteinuria, vaginal pain.
Immune system disorders: Anaphylactic reactions, bronchospasm, purpura and angioedema.
Single dose of cefpodoxime film-coated tablets
In reported clinical trials using a single dose of cefpodoxime proxetil film-coated tablets, patients were treated with the recommended dosage of cefpodoxime (200 mg). There were no deaths or permanent disabilities considered to be related to drug toxicity in these studies. Adverse events considered as possibly or probably related to cefpodoxime in single-dose clinical trials conducted by the innovator were as follows:
Incidence greater than 1%:
Nausea: 1.4%
Diarrhea: 1.2%
Incidence less than 1%:
Central Nervous System: Dizziness, headache, syncope.
Dermatologic: Rash.
Genital: Vaginitis.
Gastrointestinal: Abdominal pain.
Psychiatric: Anxiety
Most of these abnormalities were transient and not clinically significant.
The additional adverse events as per the EMC data were as follows:
Gastrointestinal disorders: Common: Gastric pressure bloody diarrhea can occur as a symptom of enterocolitis. Bloody diarrhea can occur as a symptom of enterocolitis.
Metabolism and nutrition disorders: Common: Loss of appetite.
Immune system disorders: Hypersensitivity reactions of all degrees of severity have been observed. Very rare immune system disorders include; anaphylactic reactions, bronchospasm, purpura and angioedema.
Hepatobiliary disorders: Rare: Transient moderate elevations of ASAT, ALAT and alkaline phosphatase and/or bilirubin. These laboratory abnormalities which may be explained by the infection, may rarely exceed twice the upper limit of the named range and elicit a pattern of liver injury, usually cholestatic and most often asymptomatic. Very rare: liver damage.
Skin and subcutaneous tissue disorders: Very rare: Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme.
Post-marketing Experience
The following serious adverse experiences have been reported: allergic reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme and serum sickness-like reactions, pseudomembranous colitis, bloody diarrhoea with abdominal pain, ulcerative colitis, rectorrhagia with hypotension, anaphylactic shock, acute liver injury, in utero exposure with miscarriage, purpuric nephritis, pulmonary infiltrate with eosinophilia, and eyelid dermatitis.
One death was attributed to pseudomembranous colitis and disseminated intravascular coagulation.
Cephalosporin Class Labelling
In addition to the adverse reactions listed above which have been observed in patients treated with cefpodoxime proxetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics:
Adverse Reactions and Abnormal Laboratory Tests:
Renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anaemia, hemolytic anaemia, serum sickness-like reaction, hemorrhage, agranulocytosis, and pancytopenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Reporting of side effects or suspected adverse reactions: If you experience any side effects, talk to your doctor or If you experience any side effects, talk to your doctor or pharmacist or report to indiadrugsafety@akums.in or report side effects directly by calling on the toll-free number 829 700 0060 or directly via the National Pharmacovigilance Program of India. By reporting side effects, you can help provide more information on the safety of this product.
In the event of serious toxic reaction from overdosage, haemodialysis or peritoneal dialysis may aid in the removal of cefpodoxime from the body, particularly if renal function is compromised. The toxic symptoms following an overdose of beta-lactam antibiotics may include nausea, vomiting, epigastric distress, and diarrhoea. In cases of overdosage, particularly in patients with renal insufficiency, encephalopathy may occur. The encephalopathy is usually reversible once cefpodoxime plasma levels have fallen.
Mechanism of Action
Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Cefpodoxime proxetil is an orally administered, extended-spectrum, semi-synthetic antibiotic of the cephalosporin class. Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis.
Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Microbiology
The bactericidal action of cefpodoxime results from inhibition of cell wall synthesis. Cefpodoxime is active against a wide-spectrum of Gram-positive and Gram-negative bacteria.
Cefpodoxime has shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections.
Antibacterial Spectrum:
Pharmacokinetic properties
Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. There is minimal metabolism of cefpodoxime in vivo.
Effects of Food: The extent of absorption (mean AUC) and the mean peak plasma concentration increased when film-coated tablets were administered with food. Following a 200 mg tablet dose taken with food, the AUC was 21 to 33% higher than under fasting conditions, and the peak plasma concentration averaged 3.1 mcg/mL in fed subjects versus 2.6 mcg/mL in fasted subjects. Time to peak concentration was not significantly different between fed and fasted subjects. When a 200 mg dose of the suspension was taken with food, the extent of absorption (mean AUC) and mean peak plasma concentration in fed subjects were not significantly different from fasted subjects, but the rate of absorption was slower with food (48% increase in Tmax).
Over the recommended dosing range (100 to 400 mg), the rate and extent of cefpodoxime absorption exhibited dose-dependency; dose-normalized Cmax and AUC decreased by up to 32% with increasing dose. Over the recommended dosing range, the Tmax was approximately 2 to 3 hours and the T1/2 ranged from 2.09 to 2.84 hours. Mean Cmax was 1.4 mcg/mL for the 100 mg dose, 2.3 mcg/mL for the 200 mg dose, and 3.9 mcg/mL for the 400 mg dose. In patients with normal renal function, neither accumulation nor significant changes in other pharmacokinetic parameters were noted following multiple oral doses of up to 400 mg Q 12 hours.
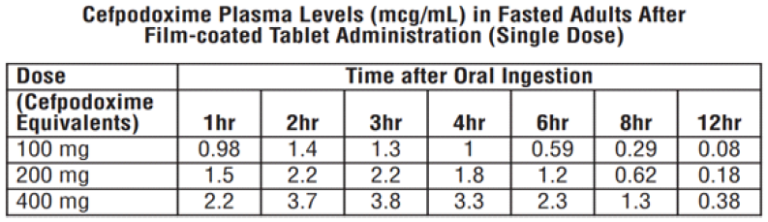
Well distributed after oral administration. Cefpodoxime reaches therapeutic concentrations in respiratory tract and genito-urinary tracts and bile. Protein binding of cefpodoxime ranges from 20 to 30%. The plasma half-life of cefpodoxime is about 2 to 3 hours and is prolonged in patients with impaired renal function. Cefpodoxime is excreted unchanged in urine.
Special Population
Renal Impairment
Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance). In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour hemodialysis procedure.
Hepatic Impairment
Absorption was somewhat diminished and elimination unchanged in patients with cirrhosis. The mean cefpodoxime T1/2 and renal clearance in cirrhotic patients were similar to those derived in studies of healthy subjects. Ascites did not appear to affect values in cirrhotic subjects. No dosage adjustment is recommended in this patient population.
Geriatrics
Elderly subjects do not require dosage adjustments unless they have diminished renal function.
Not known.
WhatsApp us
